While Medicare Part D coverage has changed, XPHOZAH is available. Learn more

42%
of patients are unable to achieve target serum phosphorus levels in any given month2
70%
of patients are unable to consistently achieve and maintain* target serum phosphorus levels over a 6-month period3
Demonstrated in chart audits between 2019 and 2023 among patients on dialysis who were also taking phosphate binders. Chart audits each year include approximately 1000 patients. Target serum phosphorus levels were defined as ≤5.5 mg/dL.3
*
At least one serum phosphorus level was >5.5 mg/dL in the 6-month evaluation period.
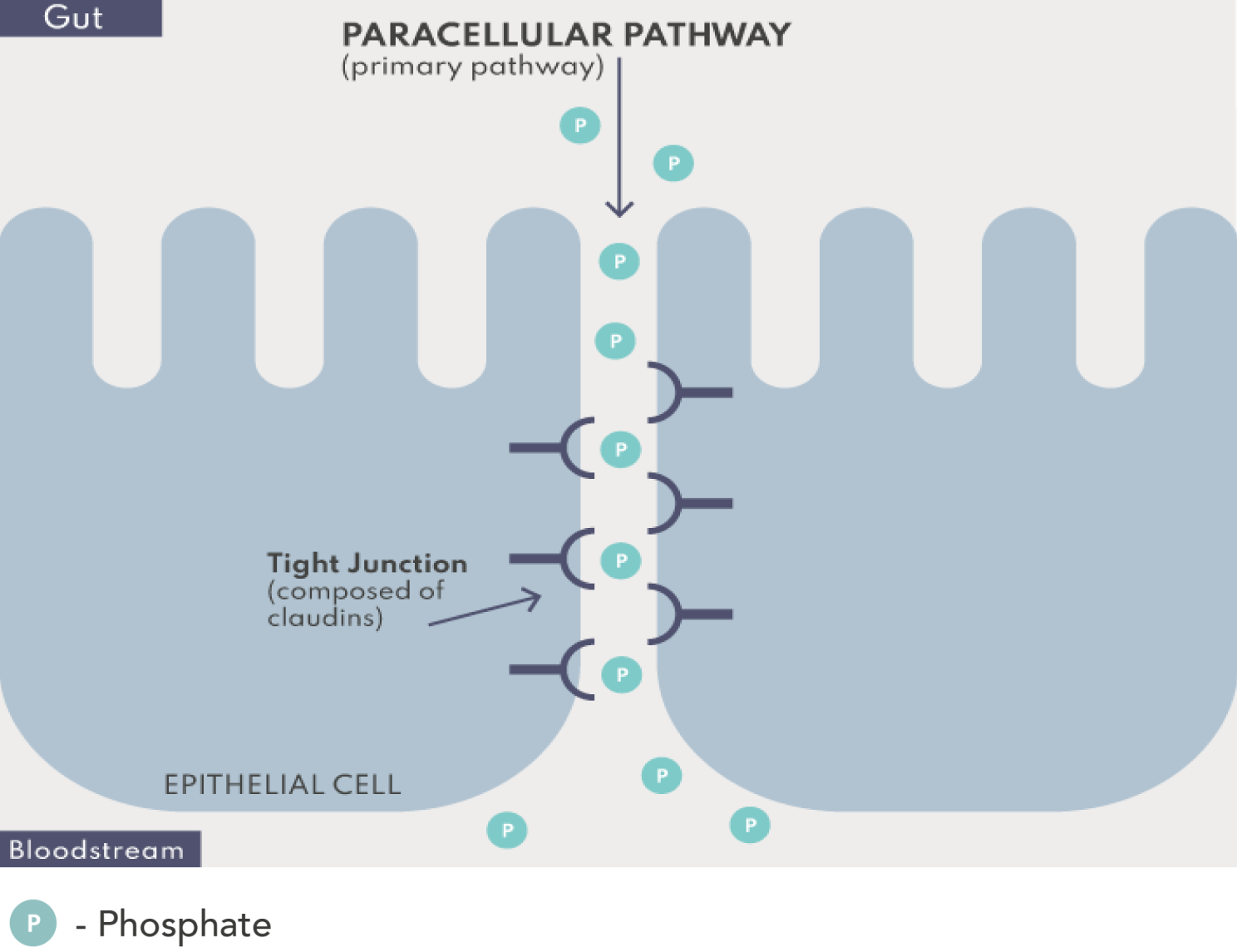
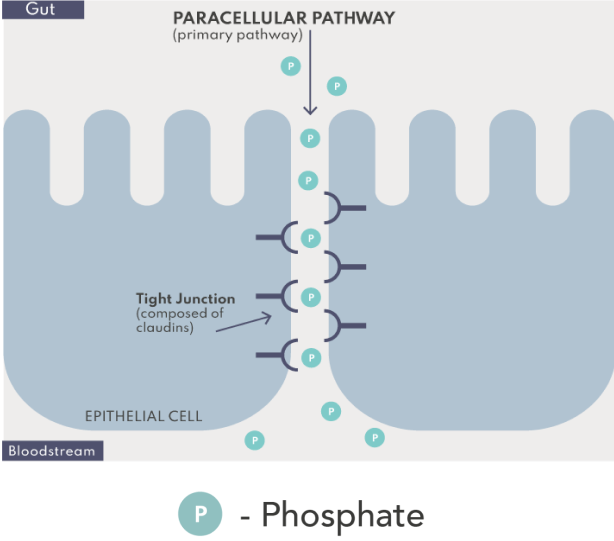
Paracellular phosphate absorption occurs passively along concentration gradients through tight junction complexes between epithelial cells.1,4,5
The paracellular pathway is responsible for the bulk of dietary phosphate absorption and does not appear to saturate.1,4,5
Pinch to Zoom
Fishbane SN, Nigwekar S. Phosphate absorption and hyperphosphatemia management in kidney disease: a physiology-based review. Kidney Med. 2021;3(6):1057-1064.
Robinson J, Hurtado T. The true state of hyperphosphatemia management in dialysis. ePoster presented at: American Society of Nephrology (ASN) Kidney Week 2020; October 22–25, 2020.
Data on File. Ardelyx, Inc. 2023.
Fouque D, Vervloet M, Ketteler M. Targeting gastrointestinal transport proteins to control hyperphosphatemia in chronic kidney disease. Drugs. 2018;78(12):1171-1186.
Saurette M, Alexander RT. Intestinal phosphate absorption: the paracellular pathway predominates? Exp Biol Med (Maywood). 2019;244(8):646-654.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
![]()
![]()
![]()