While Medicare Part D coverage has changed, XPHOZAH is available. Learn more


 Salvatore Chillemi
Salvatore ChillemiDr. Salvatore Chillemi discusses his clinical experience with XPHOZAH.

North Georgia Kidney Specialists
Atlanta, GA

 Salvatore Chillemi
Salvatore ChillemiDr. Salvatore Chillemi discusses his clinical experience with XPHOZAH.

 Rory Pace
Rory PaceBoard-certified renal nutrition specialist Rory Pace provides key insight on phosphorus management and how XPHOZAH fits into patient care discussions.

 Nelson Kopyt
Nelson KopytDrs. Nelson Kopyt and William Raffo discuss their phosphorus targets and how XPHOZAH changed their approach to managing patients above goal.

 Vincent Carsillo II
Vincent Carsillo IIDrs. Vincent Carsillo and Nelson Kopyt share their clinical perspectives on identifying patients who may benefit from XPHOZAH.

 Stuart M. Sprague
Stuart M. SpragueDr. Stuart Sprague describes our new mechanistic understanding of phosphate absorption.

 Glenn Chertow
Glenn ChertowDr. Glenn Chertow explains how we may be underestimating the portion of patients with uncontrolled hyperphosphatemia.

 Nelson Kopyt
Nelson KopytDr. Nelson Kopyt explains the crucial role of the paracellular pathway in phosphate absorption.

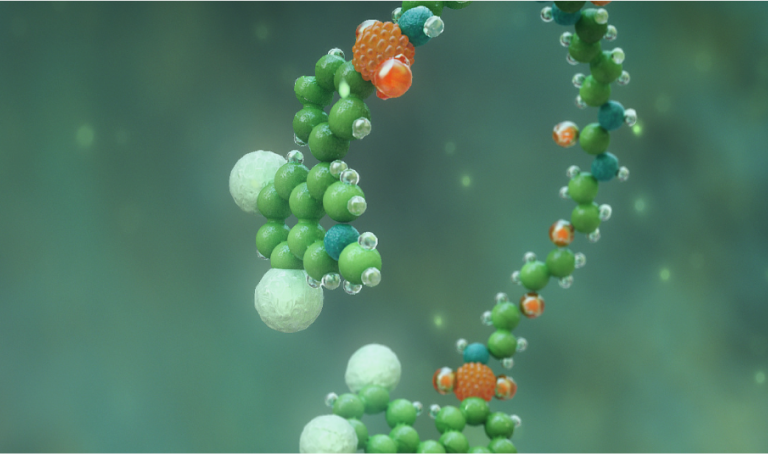 MOA
MOA
Learn how XPHOZAH works to specifically block paracellular phosphate absorption.

 Nelson Kopyt
Nelson KopytDr. Nelson Kopyt outlines why XPHOZAH should be considered as a treatment option for patients with CKD on dialysis.

 Andrew Lazar
Andrew LazarDr. Andrew Lazar explains the recommended 30 mg BID dosing for XPHOZAH.

 William Raffo
William RaffoDr. William Raffo explains why XPHOZAH is recommended to be taken before the first and last meals of the day.

 Vincent Carsillo II
Vincent Carsillo IIDr. Vincent Carsillo discusses findings that support using XPHOZAH for patients with an inadequate response to phosphate binders.

 Andrew Lazar
Andrew LazarDr. Andrew Lazar explains why some patients may experience diarrhea while using XPHOZAH.

 Andrew Lazar
Andrew LazarDr. Andrew Lazar explains how to manage diarrhea while on XPHOZAH and what to expect while on treatment.
Experts are paid advisors of Ardelyx, Inc.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
![]()
![]()
![]()