While Medicare Part D coverage has changed, XPHOZAH is available. Learn more

XPHOZAH is minimally absorbed and works via local inhibition of the sodium/hydrogen exchanger 3 (NHE3) in the GI tract to specifically block paracellular phosphate absorption.1

Blocking mechanism and duration of action enable XPHOZAH to be dosed as one 30 mg pill BID.1
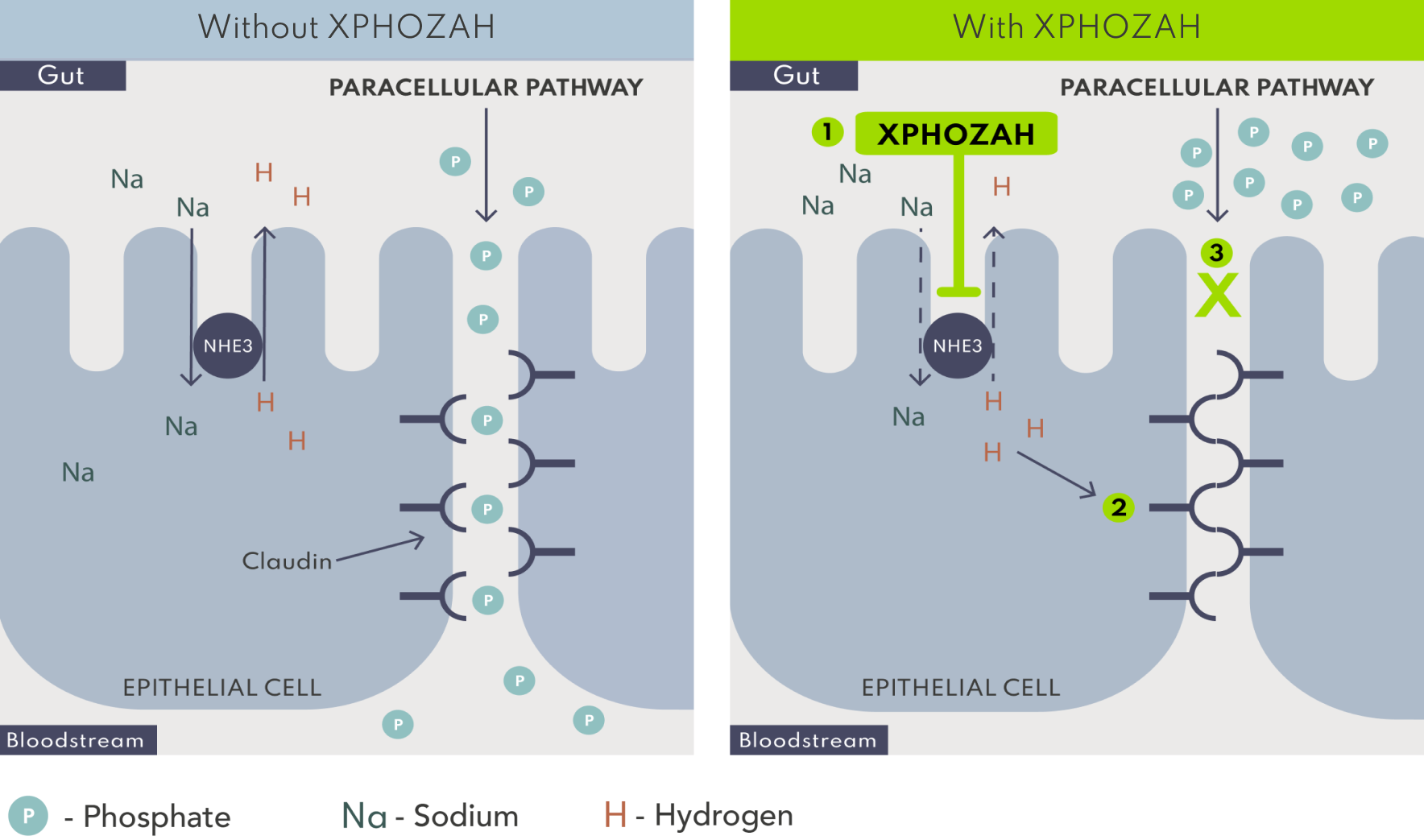
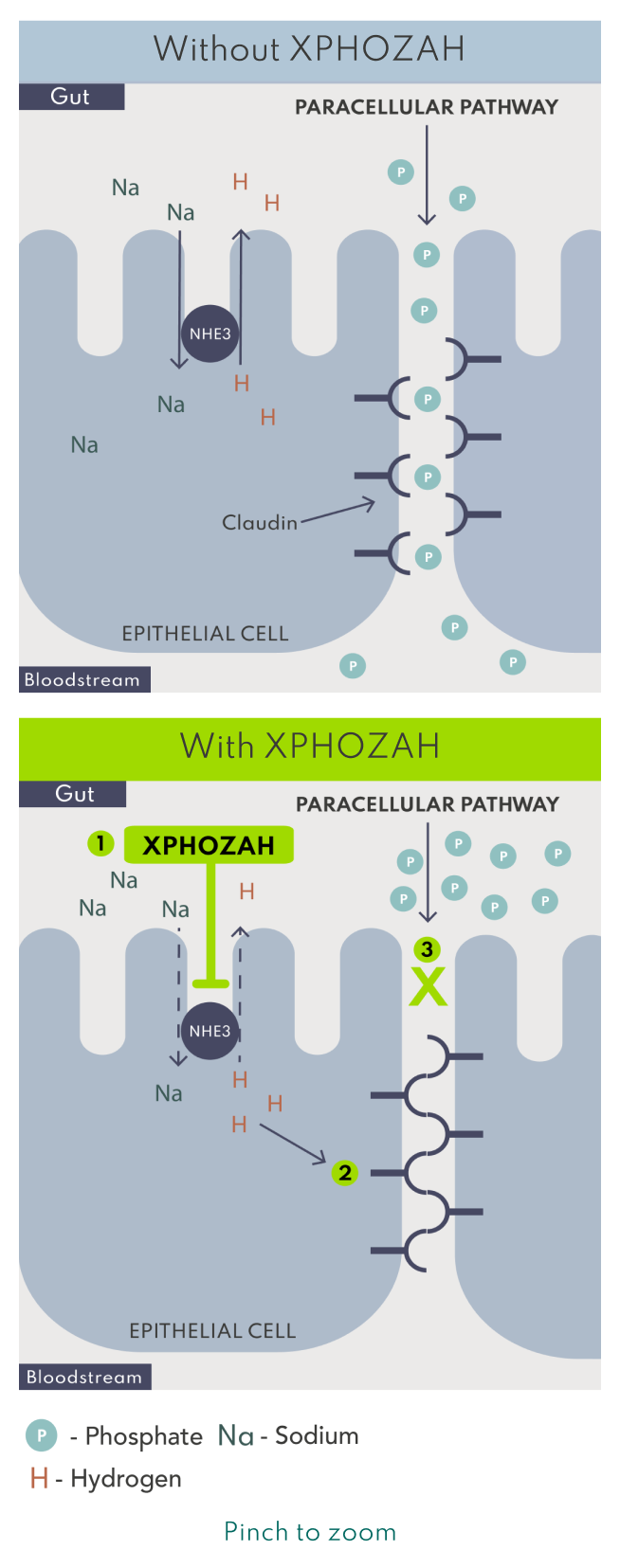
*Based on in vitro models and the relevance to humans is not known.4
GI = gastrointestinal; NHE3 = sodium/hydrogen exchanger 3.
XPHOZAH® (tenapanor) full Prescribing Information. Waltham, MA: Ardelyx, Inc.; 2023.
Kovesdy CP, Adebiyi A, Rosenbaum D, Jacobs JW, Quarles LD. Novel treatments from inhibition of the intestinal sodium-hydrogen exchanger 3. Int J Nephrol Renovasc Dis. 2021;14:411-420.
Block GA, Bleyer AJ, Silva AL, et al. Safety and efficacy of tenapanor for long-term serum phosphate control in maintenance dialysis: a 52-week randomized phase 3 trial (PHREEDOM). Kidney360. 2021;2(10):1600-1610.
King AJ, Siegel M, He Y, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018;10(456):eaam6474.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
![]()
![]()
![]()