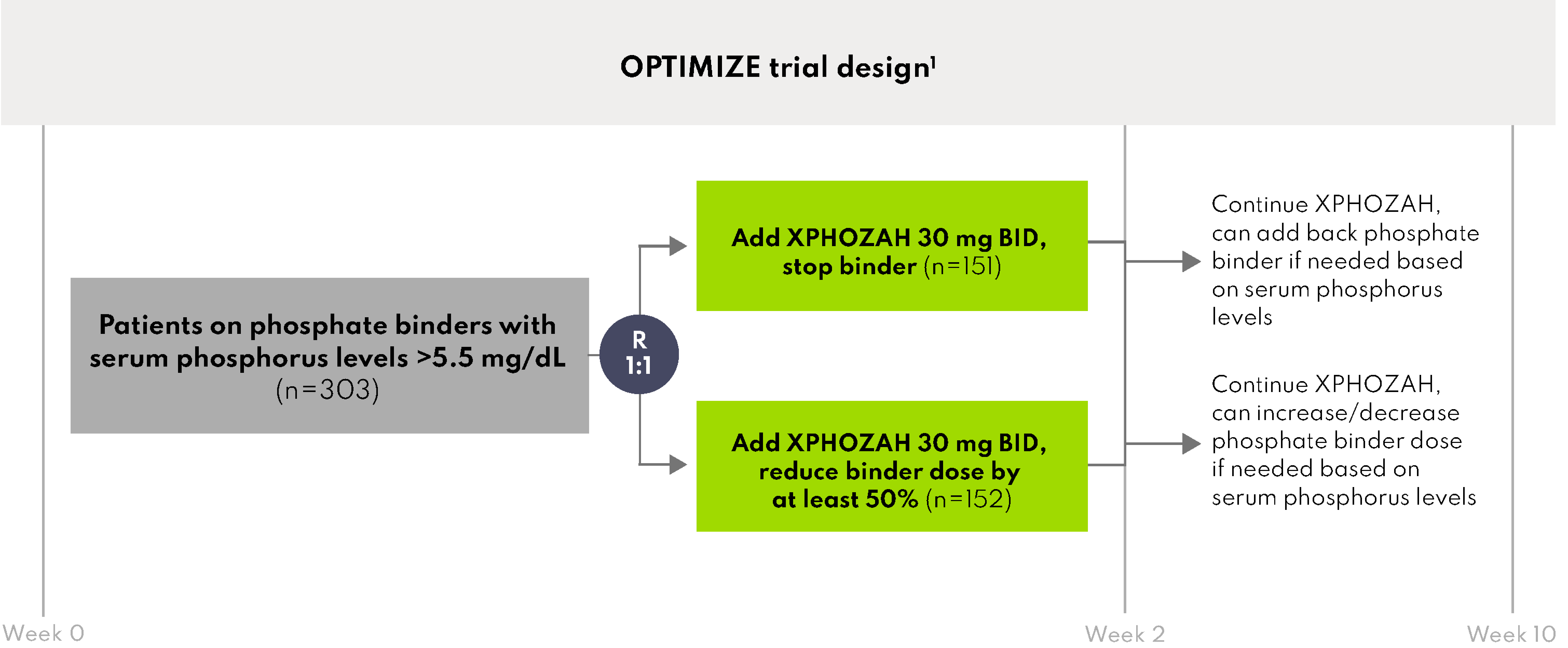
Primary Endpoint Results1
XPHOZAH Initiation Arm 1: Added XPHOZAH, stopped binder
- 34% of patients achieved serum phosphorus ≤5.5 mg/dL at week 10 compared to 0% at baseline
XPHOZAH Initiation Arm 2: Added XPHOZAH, reduced binder
- 38% of patients achieved serum phosphorus ≤5.5 mg/dL at week 10 compared to 0% at baseline
Additional Results1
XPHOZAH Initiation Arm 1: Added XPHOZAH, stopped binder
- Mean serum phosphorus reduction of 0.9 mg/dL at week 10 compared to baseline
- Median number of daily phosphate lowering pills was reduced from 9 at baseline to 5 at week 10
XPHOZAH Initiation Arm 2: Added XPHOZAH, reduced binder
- Mean serum phosphorus reduction of 1.0 mg/dL at week 10 compared to baseline
- Median number of daily phosphate lowering pills was reduced from 9 at baseline to 7 at week 10
BID = twice daily; R = randomized.
Safety1
XPHOZAH Initiation Arm 1: Added XPHOZAH, stopped binder
- 39% of patients experienced diarrhea and 7% discontinued XPHOZAH due to diarrhea
XPHOZAH Initiation Arm 2: Added XPHOZAH, reduced binder
- 42% of patients experienced diarrhea and 7% discontinued XPHOZAH due to diarrhea