While Medicare Part D coverage has changed, XPHOZAH is available. Learn more

Geoffrey A. Block, Anthony J. Bleyer, Arnold L. Silva, Daniel E. Weiner, Robert I. Lynn, Yang Yang, David P. Rosenbaum, and Glenn M. Chertow; on behalf of the PHREEDOM Trial Investigators
As published in Kidney 360.
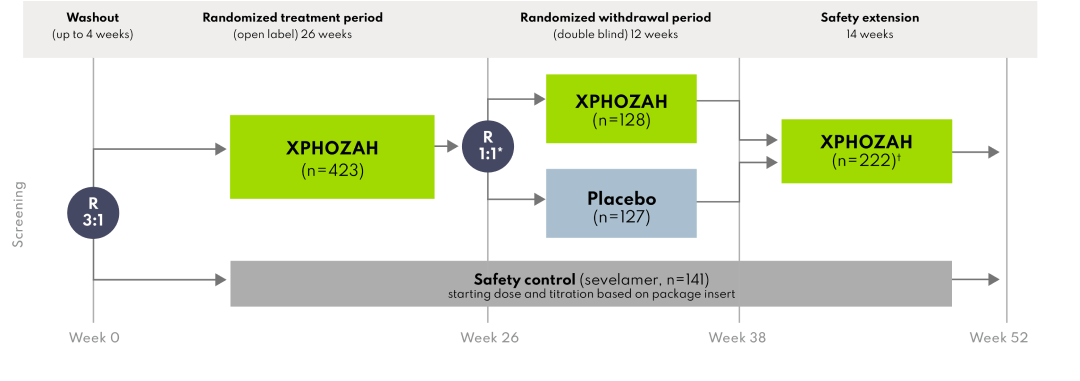
PHREEDOM included a 26-week, active-controlled, open-label, randomized treatment period, followed by a 12-week, double-blind, placebo-controlled, randomized withdrawal period.
A total of 564 patients were randomized into the 26-week treatment period (423 to XPHOZAH and 141 to the safety control arm). Among the 423 patients randomized to XPHOZAH 30 mg BID, 255 patients (60%) completed the 26-week treatment period and were re-randomized 1:1 to remain on XPHOZAH (n=128) or receive placebo (n=127).2
Pinch to zoom.
*
167 patients discontinued from the study.
†
Includes all eligible patients who completed the randomized withdrawal period from either the XPHOZAH or placebo arm.
During the randomized withdrawal period, the serum phosphorus concentration rose in the placebo group by 0.7 mg/dL (P=0.002) relative to patients who remained on XPHOZAH.1
56% of patients from the full analysis set who were part of the randomized withdrawal period met the prespecified responder definition of a serum phosphorus reduction ≥1.2 mg/dL at the end of the randomized treatment period on XPHOZAH.4
During the randomized withdrawal period, the serum phosphorus concentration rose in the placebo group by 1.4 mg/dL (P<0.001) relative to patients who remained on XPHOZAH.2
BID = twice daily; HD = hemodialysis; PD = peritoneal dialysis; PTH = parathyroid hormone; R = randomized.
Geoffrey A. Block, David P. Rosenbaum, Andrew Yan, and Glenn M. Chertow
As published in the Journal of the American Society of Nephrology.
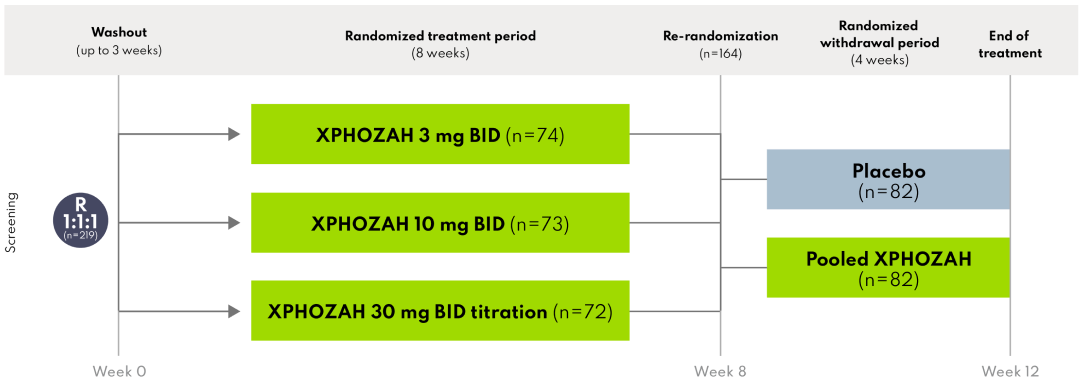
BLOCK included an 8-week, randomized, double-blind treatment period that evaluated three dosing regimens of XPHOZAH (3 mg twice daily, 10 mg twice daily, or a 30 mg twice daily titration regimen).
This was followed by a 4-week, double-blind, placebo-controlled, randomized withdrawal period during which patients were re-randomized 1:1 to their current XPHOZAH treatment or to placebo. Of the 219 patients included in the trial, 164 patients (75%) completed the 8-week randomized treatment period on XPHOZAH and were re-randomized 1:1 to remain on XPHOZAH (n=82) or receive placebo (n=82).
Pinch to zoom.
During the randomized withdrawal period, the serum phosphorus concentration rose in the placebo group by 0.7 mg/dL (P=0.003) relative to patients who remained on XPHOZAH.1
49% of patients from the full analysis set who were part of the randomized withdrawal period met the prespecified responder definition of a serum phosphorus reduction ≥1.2 mg/dL at the end of the randomized treatment period on XPHOZAH.6
During the randomized withdrawal period, the serum phosphorus concentration rose in the placebo group by 0.8 mg/dL (P=0.01) relative to patients who remained on XPHOZAH.5
Incidence of diarrhea and discontinuation due to diarrhea are shown for patients started on the 30 mg BID dose.5
BID = twice daily; HD = hemodialysis; PTH = parathyroid hormone; R = randomized.
Pablo E. Pergola, David P. Rosenbaum, Yang Yang, and Glenn M. Chertow
As published in Journal of the American Society of Nephrology.
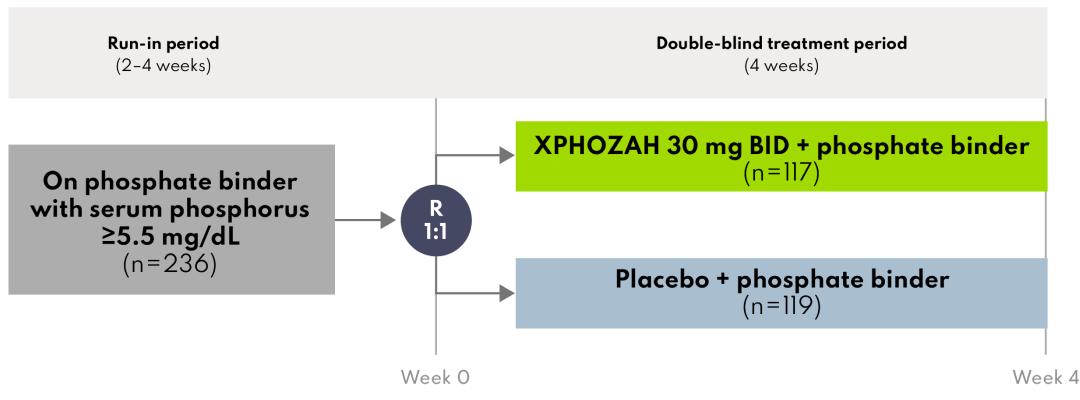
AMPLIFY was a randomized, parallel-group, double-blind, placebo-controlled study that evaluated the effect of XPHOZAH on the change in serum phosphorus when used as add-on therapy in patients on stable phosphate-binder therapy with serum phosphorus ≥5.5 mg/dL.
A total of 236 patients were randomized to receive XPHOZAH (n=117) or placebo (n=119) BID for 4 weeks.
Pinch to zoom.
BID = twice daily; CI = confidence interval; HD = hemodialysis; PD = peritoneal dialysis; PTH = parathyroid hormone.
XPHOZAH® (tenapanor) full Prescribing Information. Waltham, MA: Ardelyx, Inc.; 2023.
Block GA, Bleyer AJ, Silva AL, et al. Safety and efficacy of tenapanor for long-term serum phosphate control in maintenance dialysis: a 52-week randomized phase 3 trial (PHREEDOM). Kidney360. 2021;2(10):1600-1610.
Data on file. Ardelyx, Inc. 2020.
Data on file. Ardelyx, Inc. 2020.
Block GA, Rosenbaum DP, Yan A, Chertow GM. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019;30(4):641-652.
Data on file. Ardelyx, Inc. 2023.
Pergola PE, Rosenbaum DP, Yang Y, Chertow GM. A randomized trial of tenapanor and phosphate binders as a dual-mechanism treatment for hyperphosphatemia in patients on maintenance dialysis (AMPLIFY). J Am Soc Nephrol. 2021;32(6):1465-1473.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
XPHOZAH (tenapanor) 30 mg BID is indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis as add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy.
XPHOZAH is contraindicated in:
Patients may experience severe diarrhea. Treatment with XPHOZAH should be discontinued in patients who develop severe diarrhea.
Diarrhea, which occurred in 43-53% of patients, was the only adverse reaction reported in at least 5% of XPHOZAH-treated patients with CKD on dialysis across trials. The majority of diarrhea events in XPHOZAH-treated patients were reported to be mild-to-moderate in severity and resolved over time, or with dose reduction. Diarrhea was typically reported soon after initiation but could occur at any time during treatment with XPHOZAH. Severe diarrhea was reported in 5% of XPHOZAH-treated patients in these trials.
For additional safety information, please see full Prescribing Information.
![]()
![]()
![]()